Chemical Tests for the Detection of Elements in Organic Compounds
When a new organic compound is discovered, one of the first steps in its analysis is to identify the elements present in it. Organic compounds mainly contain carbon and hydrogen, but they may also include nitrogen, sulphur, and halogens.
To detect these elements, chemists use a series of systematic chemical tests, which form an important part of organic qualitative analysis.In this blog, we will discuss the chemical tests used for the detection of carbon, hydrogen, nitrogen, sulphur, and halogens in organic compounds.
Testing for Carbon and Hydrogen
Carbon and hydrogen are essential components of organic compounds. Their presence is confirmed by oxidising the compound and identifying the products formed.
Principle
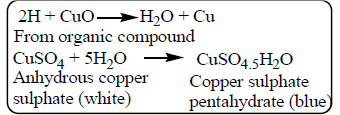
When an organic compound is heated with an oxidising agent, carbon is converted into carbon dioxide and hydrogen is converted into water.
Test Observations
- Anhydrous copper sulphate changes from white to blue, indicating the presence of water and hence hydrogen.
- Lime water turns milky, indicating the presence of carbon dioxide and hence carbon.
Conclusion
The formation of water and carbon dioxide confirms the presence of hydrogen and carbon in the organic compound.
Method for Testing Carbon and Hydrogen
Procedure
- A small amount of the organic compound is mixed with dry copper(II) oxide in a ratio of approximately 1 : 3.
- The mixture is heated strongly in a dry test tube.
- Carbon in the compound is oxidised to carbon dioxide, and hydrogen is oxidised to water.
- The gases produced are passed first through anhydrous copper sulphate and then through lime water.
Result
- Blue colour of copper sulphate → hydrogen present
- Milky lime water → carbon present
Detection of Nitrogen, Sulphur, and Halogens
(Lassaigne’s Test)
Unlike carbon and hydrogen, elements such as nitrogen, sulphur, and halogens cannot be detected directly. For this purpose, the organic compound is first converted into an ionic form using sodium metal.
Preparation of Sodium Fusion Extract (Lassaigne’s Extract
Steps Involved
- A small piece of freshly cut sodium metal is heated in a fusion tube.
- A small quantity of the organic compound is added to the tube.
- The mixture is heated strongly until the tube becomes red hot.
- The red-hot tube is plunged into a china dish containing distilled water.
- The tube breaks, and the contents are boiled gently.
- The solution is filtered.
Result
The clear filtrate obtained is called sodium fusion extract or Lassaigne’s extract. This extract is used for further tests.
Detection of Sulphur
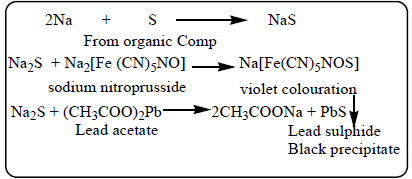
Sulphur present in the organic compound is converted into sodium sulphide during sodium fusion.
Observation
- Formation of a black precipitate or a violet colour confirms the presence of sulphur.
Conclusion
Sulphur is present in the organic compound.
Detection of Halogens
Halogens (chlorine, bromine, iodine) are converted into sodium halides during fusion.
Observation
- Formation of a white, pale yellow, or yellow precipitate after treatment with silver nitrate confirms the presence of halogens.
Conclusion
The colour of the precipitate helps identify the specific halogen present.
Detection of Nitrogen and Sulphur Together
When both nitrogen and sulphur are present, sodium fusion converts them into sodium thiocyanate.
Observation
- Development of a blood-red colour indicates the presence of both nitrogen and sulphur.
Detection of Nitrogen
Nitrogen in the organic compound is converted into sodium cyanide during fusion.
Observation
- Formation of a blue or green precipitate confirms the presence of nitrogen.
Summary
Chemical tests play a crucial role in the elemental analysis of organic compounds.
- Carbon and hydrogen are detected by oxidation.
- Nitrogen, sulphur, and halogens are detected using Lassaigne’s test.
A clear understanding of these tests helps students write accurate, well-structured answers in board examinations and strengthens their foundation in organic chemistry.